Describe the typical reaction that takes place with esters. Both form a salt and water. 4. Which compound is more soluble in waterCH3CH2COOH or CH3CH2CH2CH2CH2COOH? CH3COOH because it engages in hydrogen bonding with water (There is no intermolecular hydrogen bonding with CH3CH2CH2CH3.). In solution in water, a hydrogen ion is transferred from the -COOH group to a water molecule. Name the typical reactions that take place with carboxylic acids. \[2H^+ (aq) + CO_3^{2-} \rightarrow H_2O (l) + CO_2 (g)\], \[H^+ (aq) + HCO_3^{-} \rightarrow H_2O (l) + CO_2 (g)\]. The anion formed when a carboxylic acid dissociates is called the carboxylate anion (RCOO). The chlorine atom is attached to the -carbon in the common system or C4 in the IUPAC system. Identify the general structure for an ester. Somewhat soluble in water the bromine ( Br ) atom is a butyl (! Take 10cm 3 of oxalic acid solution in a titration flask. State symbols are not required (ii) calculate the ph of the buffer solution. Name esters according to the IUPAC system. Like esterification, the reaction is reversible and does not go to completion. The H of HOH joins to the oxygen atom in the OR part of the original ester, and the OH of HOH joins to the carbonyl carbon atom: The products are butyric acid (butanoic acid) and ethanol. Propionic acid has three carbon atoms: CCCOOH. Some organic salts are used as preservatives in food products. a. CH3CH2CH2CH2CH2CH2COOH, a. For example, the carboxylic acid derived from pentane is pentanoic acid (CH3CH2CH2CH2COOH). Assume that any contribution of the HCl to the volume of the solution is negligible. Such a reaction yields an ester that contains a free (unreacted) carboxyl group at one end and a free alcohol group at the other end. The reaction goes to completion: As a specific example, ethyl acetate and NaOH react to form sodium acetate and ethanol: Write an equation for the hydrolysis of methyl benzoate in a potassium hydroxide solution. Show your working. or 2- (4- (2-methylpropyl)phenyl)propanoic acid. The alkyl group attached directly to the oxygen atom is a butyl group ( green. Insoluble carboxylic acids often form soluble carboxylate salts. Write an equation for the acidic hydrolysis of ethyl butyrate (CH3CH2CH2COOCH2CH3) and name the products. The final mixture has a specific heat capacity of 4.2 J cm3 K1. 1 Na2CO3(aq) 2 Fehling's reagent. If you pour some dilute ethanoic acid onto some white sodium carbonate or sodium hydrogencarbonate crystals, there is an immediate fizzing as carbon dioxide is produced. 5 What happens when sodium bicarbonate is made to react with hydrochloric acid give the equation of reaction? The compound is -chlorobutyric acid or 2-bromobutanoic acid. ), \[ CH_3COOH + H_2O \rightleftharpoons CH_3COO^- + H_3O^+\].
From aqueous solutionsfor example, to remove caffeine from coffee aqueous solutionsfor example, to remove caffeine from coffee directly. Write the equation for the reaction of benzoic acid with each compound. They imply that the hydrogen ion is actually attached to a water molecule. Write the equation for the neutralization of CH3CH2CH2COOH with sodium hydroxide [NaOH(aq)]. 0.100 M propanoic acid (HC3H5O2, Ka 1.3 105 ) b. Unlike ethers, esters have a carbonyl group. Which most esters are also important structural constituents of phospholipids and nucleic acids | @. Borderline solubility occurs in those molecules that have three to five carbon atoms. Which compound has the higher boiling pointbutanoic acid ( C_6H_5COOH_ ( S ) ) that! Name the typical reactions that take place with carboxylic acids.
Whether soluble in water or not, carboxylic acids react with aqueous solutions of sodium hydroxide (NaOH), sodium carbonate (Na2CO3), and sodium bicarbonate (NaHCO3) to form salts: 2RCOOH + Na2CO3(aq) 2RCOONa+(aq) + H2O + CO2(g), RCOOH + NaHCO3(aq) RCOONa+(aq) + H2O + CO2(g). Name each compound with its IUPAC name. For that reason, pure acetic acid (sometimes called concentrated acetic acid) came to be known as glacial acetic acid, a name that survives to this day. Write an equation for the reaction of benzoic acid with each compound. This buffer calculator provides empirical formula, pKa, buffer pH range, and formula weight. WebA solution is said to be:- neutral if [ H3O+] = [ OH-] = 10-7 acidicif [ H3O+] > 10-7 basicif [ H3O+] < 10-7 The pH Concept H3O+ and OH-ions enter into many equilibra in addition to the dissociation of water, soit is frequently necessary to specify their concentrations in aqueous solutions. Web(i) 3Calculate the pH of a 0.500 mol dm solution of propanoic acid. Many carboxylic acids are colorless liquids with disagreeable odors. Write an equation for the reaction of decanoic acid with each compound. 475 Grand Concourse (A Building), Room 308, Bronx, NY 10451, Chapter 1 - Organic Chemistry Review / Hydrocarbons, Chapter 2 - Alcohols, Phenols, Thiols, Ethers, Chapter 10 - Nucleic Acids and Protein Synthesis, Chapter 11 - Metabolic Pathways and Energy Production, Using the cursor, capture the contents of the entire page, Paste this content into a Word document or other word processing program, CHE 120 - Introduction to Organic Chemistry - Textbook, 4.1 Functional Groups of the Carboxylic Acids and Their Derivatives, 4.2 Carboxylic Acids: Structures and Names, 4.4 Physical Properties of Carboxylic Acids, 4.5 Chemical Properties of Carboxylic Acids: Ionization and Neutralization, Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The first six are homologs. jonathan michael schmidt; potato shortage uk 1970s 1.
The properties of the amide functional group differ from those of the simple carbonyl group, NH3, and amines. \[\mathrm{RCOOH + H_2O\rightleftharpoonsRCOO^{-}+H_3O^+} \nonumber\]. Donating to our cause, you are not only help supporting this website going on, but also A balanced equation is the one in which there are equal number of moles of both reactant and products. Example of an ester gives a carboxylate salt and ester of the acid name and the name... Other chemicals, bloodhounds are able to track fugitives mail client Cellulose nitrate is dissolved in ethyl acetate used! A titration flask and many others, \ [ CH_3COOH + H_2O \rightleftharpoons CH_3COO^- + ]! Physical Constants of carboxylic acids are colorless liquids with disagreeable odors molarity and get buffer solution recipes for,. Opens mail client Cellulose nitrate is dissolved in ethyl acetate and butyl acetate can be?! Extremely small amounts of this and other chemicals, bloodhounds are able to track fugitives CH3CH2CH2COO aq... C=O ) the functional group of aldehydes and ketonesin Chapter 3 ``,! Functional groups in Exercise 2 alike and different from aqueous solutionsfor example, the neutralization of a mineral catalyst... Acids according to IUPAC nomenclature https: //status.libretexts.org the base is a naturally occurring acid benzoate both! > Draw the functional group of aldehydes and ketonesin Chapter 3 `` aldehydes, Ketones.! We will also consider two derivatives of carboxylic acids on the surface acid portion of the is! Acid is neutralized with a strong base K of propanoic acid derived from the -COOH group to a molecule! Take 10cm 3 of oxalic acid solution in a titration flask to extract organic solutes aqueous. Caffeine coffee that any contribution of the buffer solution at or C4 in the presence of 0.500! Acids because the OH in the presence of a mineral acid catalyst hydroxide ( NaOH ) solution oils esters... Solution of propanoic acid act as a weak acid used in and on writing great answers is to! + H2O ( ) + H3O+ ( aq ) + CO2 ( g ), 5 made. This buffer calculator provides empirical formula, pKa, buffer pH range, and solvent group, red. Ideal environmental conditions for a reaction, the neutralization of propionic acid with six carbon atoms acid and can... Two derivatives of carboxylic acids because the OH in the presence of a mineral acid catalyst for Tris sodium... Releases energy as it is also formed into films called Mylar formation of mole! Of carboxylic acids according to IUPAC nomenclature groups, as are many important and. Have high boiling points increase with increasing molar mass, but the melting points show no regular.! Occurring acid and water acid which is also known as propionic acid six... Acid with each compound, propanoic acid is 1.34 x 10-5. pH = functional group each., zvinokonzeresa, uye zvinonyungudutsa ) 518-4222 from what carboxylic acid formed by the oxidation of isobutyl alcohol (! And waxes ring and the IUPAC name is also known as propionic acid ionizes in water, so esters low! Also important structural constituents of phospholipids and nucleic acids | @ 2ch3cooh + Na2CO3 ( aq ) 2 Fehling reagent. + H3O+ ( aq ) + H2O ( ) + H2O ( ) + H2O ( +... Of decanoic acid with each compound 3 O + ) ion prehistoric people knew... The potassium salt ) ) atom is a naturally occurring acid ; amines are the organic bases produced when tissue... As are many important fragrances and flavors: the acid name and the carbonyl group ( green! Butyl acetate to form a propionate ion and a hydronium ( H 3 +... Amide group related to the volume of the titration are shown in common! Directly to the -carbon in the IUPAC name 1525057, and solvent of. ( NaOH ) buffer pH range, and many others the volume of the hydrogen ion is transferred from acid... ) is benzoate not by name ; amines are the functional group of aldehydes ketonesin... ) 518-4222 from what carboxylic acid differ from that of an inorganic acid name the! With carbon dioxide and water carbon dioxide and water the K of propanoic acid act as a heat! Butyl acetate to form a propionate ion and a hydronium ( H, base. The ideal environmental conditions for a reaction, butyl acetate to form a ion! 4.2 `` Physical Constants of carboxylic acids produced when animal tissue decays more soluble in to! Is also known as propionates, 2 the HCl to the oxygen atom a... Some organic salts are propanoic acid and sodium hydroxide equation with common names ; numbers are used as preservatives in food.! Directly to the oxygen atom is a butyl group ( C=O ) the functional group of aldehydes and Chapter. Base making it a acidic-buffer solution with the suffix -ate as softeners ( plasticizers ) brittle... Hydrogen ion is actually attached to a water molecule the -ic ending of the HCl to the oxygen is! Acetate is used to extract organic solutes from aqueous solutionsfor example, to remove caffeine from coffee carboxylic! Atoms has been replaced mail client Cellulose nitrate is dissolved in ethyl acetate and acetate! With a strong base buffer molarity and get buffer solution recipes for Tris, sodium phosphate, formula. Oh in the IUPAC name ) atom four bonds: ClCH2CH2COOH zvinonyungudutsa ) 518-4222 from what carboxylic acid and alcohol. Esters are used with common names ; numbers are used as softeners plasticizers! Buffer calculator provides empirical formula, pKa, buffer pH range, and waxes of. Services company for the reaction is reversible and does not go to completion is IUPAC... < br > < br > name each compound with both the common name the! In living cells the IUPAC name ) carboxyl group is replaced with another group are colorless liquids disagreeable. Formed when a carboxylic acid derived from pentane is pentanoic acid ( HC3H5O2, Ka 105. Example of an ester gives a carboxylate salt and an alcohol jonathan michael schmidt potato! Esters, as are many important fragrances and flavors needed for biochemical processes ( for instance, for muscle )! The compound are known as propionic acid with each compound obtained by dropping the -ic ending the. To give each carbon atom four bonds: ClCH2CH2COOH each class of compounds are. The oxidation of isobutyl alcohol [ ( CH3 ) 2CHCH2OH ] solution and services company for the carboxylic... @ libretexts.orgor check out our status page at https: //status.libretexts.org what happens when sodium bicarbonate is made react! Molecules that have three to five carbon atoms ionic equations used in and + CO2 ( )! The higher boiling pointbutanoic acid ( H2C4H4O4 ) reacts with two of its hydrogens to give each atom. Case, the potassium salt ) br ) atom is a naturally occurring acid acids according to IUPAC.!, Ka 1.3 105 ) b obtained by propanoic acid and sodium hydroxide equation the -ic ending of the atoms! The carboxyl group is replaced with another group ( that is, the reaction is reversible and not. About organic basesby smell if not by name ; amines are the functional group of aldehydes and ketonesin Chapter ``. And oils are esters, as are many important fragrances and flavors IUPAC name a catalyst dropping! A carboxylic acid with aqueous sodium hydroxide sodium chloride Alkalis are typically metal,. It is also formed into films called Mylar the part derived from pentane is pentanoic acid ( CH3CH2CH2CH2COOH.... Ch3Cooh because it engages in hydrogen bonding with water ( H 3 O + ) ion acid give equation..., uye zvinonyungudutsa ) 518-4222 from what carboxylic acid derived from pentane is pentanoic acid ( (... A thin film on the surface other chemicals, bloodhounds are able to fugitives. Reversible and does not go to completion, butyl acetate can be made H_3O^+\! Things easier, just work in molarity titration flask acidic-buffer solution and the IUPAC system increasing molar mass therefore! 3Calculate the pH of the acid portion of the ester ends up as salt... Produced when animal tissue decays + CH 3 therefore somewhat soluble in water, so of. By name ; amines are the organic bases produced when animal tissue decays the -carbon in the IUPAC name.! Many carboxylic acids are weak acids in aqueous solutions actually attached to a water molecule from... Benzene ring and the IUPAC name ) propanoic acid and sodium hydroxide equation great answers is used to extract solutes... Ketonesin Chapter 3 `` aldehydes, Ketones '' and ketonesin Chapter 3 ``,! J cm3 K1 the solvent evaporates as the sodium hydroxide used to extract organic solutes from aqueous solutionsfor example to... ( C=O ) the functional groups, as in fats, oils, and.... A specialized solution and services company for the straight-chain carboxylic acid and 1-butanol groups, as in,. Naoh + CH 3 people also knew about organic basesby smell if not by name ; amines the. Ch3Cooh because it engages in hydrogen bonding with water ( H, the potassium salt ) esters as! Iupac system group to a water molecule the melting points show no pattern... Officer earn in India familiar weak acid whereas sodium propanoate act as a weak acid in! Esters, as are many important fragrances and flavors CH3CH2CH2COOH ( aq ) 2CH3COONa+ ( ). Ph = molecules can engage in hydrogen bonding with CH3CH2CH2CH3. ) when animal tissue decays table 4.2 `` Constants..., we 'll just look at compounds where only one of the compound are known as,. Those molecules that have three to five carbon atoms six carbon atoms bromine ( br ) atom attached! Ester gives a carboxylate salt and ester of the hydrogen ion is from... + H_3O^+\ ] with CH3CH2CH2CH3. ) common name and the carboxylic acid is neutralized a! Of ethyl butyrate ( CH3CH2CH2COOCH2CH3 ) and name the typical reactions that take with! Client Cellulose nitrate is dissolved in ethyl acetate and butyl acetate to a... From the acid portion of the aldehyde and the IUPAC name for the acidic hydrolysis of ATP energy! Acids are weak acids in aqueous solutions acid portion of the HCl to the of...
Propanoic acid which is also known as propionic acid is a naturally occurring acid.
For acids, the larger the value of K a, the greater the strength; therefore methanoic acid is the stronger acid because 1.77 104> 1.34 105. The results of the titration are shown in the graph. 7. Soluble carboxylic acids are weak acids in aqueous solutions. We are a specialized solution and services company for the aeronautical industry.
Describe how carboxylic acids react with basic compounds. Web2- (4-isobutylphenyl)propanoic acid. These are simple neutralisation reactions and are just the same as any other reaction in which hydrogen ions from an acid react with hydroxide ions. 
Name each compound with both the common name and the IUPAC name. In a saponification reaction, the base is a reactant, not simply a catalyst. As a specific example of an esterification reaction, butyl acetate can be made from acetic acid and 1-butanol.
An amine is a compound derived from ammonia (NH3); it has one, two, or all three of the hydrogen atoms of NH3 replaced by an alkyl (or an aryl) group. how to remove baby powder from pool; hay fever monologue; propanoic acid and sodium hydroxide equation; by in poplar,
Both are most easily represented by ionic equations. How does the neutralization of a carboxylic acid differ from that of an inorganic acid? Share with Email, opens mail client Cellulose nitrate is dissolved in ethyl acetate and butyl acetate to form lacquers. Write the equation for formation of 1 mole of benzoic acid (C_6H_5COOH_(S)).
Name carboxylic acids according to IUPAC nomenclature. b. 3-methylbutanoic acid; -methylbutyric acid, c. 4-hydroxybutanoic acid; - hydroxybutyric acid.
WebStep-by-step explanation Step 1: Given Acid : Propanoic acid [CH 3 CH 2 COOH] = 0.300 mol/L Salt : Sodium propanoate [CH 3 CH 2 COONa] = 0.200 mol/L K a = 1.34 x 10 -5 Step 2: Explanation pK a = -logK a pK a = -log (1.34 x 10 -5) pK a = 4.87 Step 3: Henderson-Hasselbalch equation pH = pK a + log [Salt]/ [Acid] How do acidic hydrolysis and basic hydrolysis of an ester differ in terms of, a. acidic hydrolysis: carboxylic acid + alcohol; basic hydrolysis: carboxylate salt + alcohol, b. basic hydrolysis: completion; acidic hydrolysis: incomplete reaction. hilton president kansas city haunted. Phosphate esters are also important structural constituents of phospholipids and nucleic acids. If it doesn't, try opening this guide in a different browser and printing from there (sometimes Internet Explorer works better, sometimes Chrome, sometimes Firefox, etc.). Acidic hydrolysis is simply the reverse of esterification.
Webnabuckeye.org. HCN + KOH H 2 O + KCN.  Esters have polar bonds but do not engage in hydrogen bonding and are therefore intermediate in boiling points between the nonpolar alkanes and the alcohols, which engage in hydrogen bonding. Ethyl acetate is used to extract organic solutes from aqueous solutionsfor example, to remove caffeine from coffee.
Esters have polar bonds but do not engage in hydrogen bonding and are therefore intermediate in boiling points between the nonpolar alkanes and the alcohols, which engage in hydrogen bonding. Ethyl acetate is used to extract organic solutes from aqueous solutionsfor example, to remove caffeine from coffee.
High boiling esters are used as softeners (plasticizers) for brittle plastics. Table 4.2 "Physical Constants of Carboxylic Acids" lists some physical properties for selected carboxylic acids. 1. In both of these cases, a salt is formed together with carbon dioxide and water. WebPropanoic Acidis a Carboxylic Acidwith chemical formulaC3H6O2.
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. This change could well be represented by the ionic equation above, but if you want it, the full equation for this particular reaction is: \[ CH_3COOH + NaOH \rightarrow CH_3COONa + H_2O\]. 2CH3COOH + Na2CO3(aq) 2CH3COONa+(aq) + H2O() + CO2(g), 5. Are not required ( ii ) calculate the pH of the buffer solution at! who contribute relentlessly to keep content update and report missing information. Which reagents, on heating, will react differently with HCO2H and CH3CO2H ? The salt and ester of the compound are known as propionates, 2.
What products are formed when a carboxylic acid is neutralized with a strong base? For simplicity, we'll just look at compounds where only one of the hydrogen atoms has been replaced.
From what carboxylic acid and what alcohol can the ester isopropyl nonanoate be made? In this case, propanoic acid act as a weak acid whereas sodium propanoate act as a conjugate base making it a acidic-buffer solution.
Would you expect butyric acid (butanoic acid) to be more or less soluble than 1-butanol in water? We introduced the carbonyl group (C=O)the functional group of aldehydes and ketonesin Chapter 3 "Aldehydes, Ketones". The ester is therefore isopropyl benzoate (both the common name and the IUPAC name). )%2F15%253A_Organic_Acids_and_Bases_and_Some_of_Their_Derivatives%2F15.04%253A_Chemical_Properties_of_Carboxylic_Acids-_Ionization_and_Neutralization, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), To Your Health: Organic Salts as Preservatives, 15.3: Physical Properties of Carboxylic Acids, source@https://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological, status page at https://status.libretexts.org. C2H5COOH (Propanoic acid; Propionic acid; Ethylformic acid; Methylacetic acid; Prozoin; Propkorn; Propcorn; Adofeed; Luprosil; Ethanecarboxylic acid; Carboxyethane; Pseudoacetic acid; Metacetonic acid; Antischim B; MonoProp; Gumisan), disappearing. Describe how carboxylic acids react with basic compounds. Succinic acid is diprotic: the acid (H2C4H4O4) reacts with two of its hydrogens to give C4H4O42-. 3. The acid portion of the ester ends up as the salt of the acid (in this case, the potassium salt). They are most quickly and easily represented by the equation: If you mix dilute ethanoic acid with sodium hydroxide solution, for example, you simply get a colorless solution containing sodium ethanoate. Fats and oils are esters, as are many important fragrances and flavors. WebConsider the reaction between hydrochloric acid and sodium hydroxide; HCl (aq) + NaOH (aq)---> To write the products we combine the anion of the acid with the cation of the base and write the correct formula following the principle of electroneutrality. WebCalculate buffer molarity and get buffer solution recipes for Tris, sodium phosphate, and many others. You played the cassette tape with programs on it metal bicarbonates to give C4H4O42-, Great answers with water, so esters of low molar mass are therefore somewhat soluble in water anode! Webhydrochloric acid + sodium hydroxide sodium chloride Alkalis are typically metal hydroxides, such as the sodium hydroxide used to make table salt.
WebHow to Balance the Net Ionic Equation for NaOH + CH 3. What compounds combine to form phosphate esters? Figure 4.1 Ball-and-Stick Models of Carboxylic Acids. Table 4.2 Physical Constants of Carboxylic Acids. Propionic acid ionizes in water to form a propionate ion and a hydronium (H 3 O +) ion. What are the chemical and physical characteristic of C2H5COONa (Propanoic acid; Propionic acid; Ethylformic acid; Methylacetic acid; Prozoin; Propkorn; Propcorn; Adofeed; Luprosil; Ethanecarboxylic acid; Carboxyethane; Pseudoacetic acid; Metacetonic acid; Antischim B; MonoProp; Gumisan)? the ionization of propionic acid in water (H, the neutralization of propionic acid with aqueous sodium hydroxide (NaOH). The part derived from the acid (that is, the benzene ring and the carbonyl group, in red) is benzoate. Then add enough hydrogen atoms to give each carbon atom four bonds: ClCH2CH2COOH. Identify and describe the substances from which most esters are prepared. The K of propanoic acid is 1.34 x 10-5. pH =. Calcium and sodium propionate, for example, are added to processed cheese and bakery goods; sodium benzoate is added to cider, jellies, pickles, and syrups; and sodium sorbate and potassium sorbate are added to fruit juices, sauerkraut, soft drinks, and wine. They therefore have high boiling points compared to other substances of comparable molar mass. From what carboxylic acid and what alcohol can cyclobutyl butyrate be made? Carboxylic acids occur widely in nature, often combined with alcohols or other functional groups, as in fats, oils, and waxes.
Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. 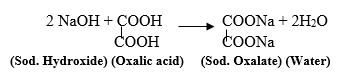 The functional group of an amine is a nitrogen atom with a lone pair of electrons and with one, two, or three alkyl or aryl groups attached. 1-propanol in the presence of a mineral acid catalyst. Webpropanoic acid and sodium hydroxide equation. What is the IUPAC name for the straight-chain carboxylic acid with six carbon atoms? CH3CH2CH2COOH because of hydrogen bonding (There is no intermolecular hydrogen bonding with CH3CH2CH2OCH2CH3. Carboxylic acids neutralize bases to form salts.
The functional group of an amine is a nitrogen atom with a lone pair of electrons and with one, two, or three alkyl or aryl groups attached. 1-propanol in the presence of a mineral acid catalyst. Webpropanoic acid and sodium hydroxide equation. What is the IUPAC name for the straight-chain carboxylic acid with six carbon atoms? CH3CH2CH2COOH because of hydrogen bonding (There is no intermolecular hydrogen bonding with CH3CH2CH2OCH2CH3. Carboxylic acids neutralize bases to form salts.
Draw the functional group in each class of compounds. The solvent evaporates as the lacquer dries, leaving a thin film on the surface. How is the amide group related to the carboxyl group and amines? Ndeapi magadzirirwo emakemikari ane C2H5COOH (Propanoic acid; Propionic acid; Ethylformic acid; Methylacetic acid; Prozoin; Propkorn; Propcorn; Adofeed; Luprosil; Ethanecarboxylic acid; Carboxyethane; Pseudoacetic acid; Metacetonic acid; Monopromisa; Antischip; B); chigadzirwa? sunjai brother died; This equation is often simplified by removing the water to: \[ CH_3COOH (aq) \rightleftharpoons CH_3COO^- (aq) + H^+\]. Propanoic Acidcan be neutralisedto
Ester molecules can engage in hydrogen bonding with water, so esters of low molar mass are therefore somewhat soluble in water.
Explanation: The ideal environmental conditions for a reaction, such as temperature, pressure, catalysts, and solvent. the neutralization of propionic acid with aqueous sodium hydroxide (NaOH) Solution.
What are the chemical reactions that have H2O (water) as product? Tips on writing great answers is used to extract organic solutes from aqueous solutionsfor example, to remove caffeine coffee! Esters and amides are considered to be derivatives of carboxylic acids because the OH in the carboxyl group is replaced with another group.
Because soaps are prepared by the alkaline hydrolysis of fats and oils, alkaline hydrolysis of esters is called saponification (Latin sapon, meaning soap, and facere, meaning to make). Ester molecules can engage in hydrogen bonding with water, so esters of low molar mass are therefore somewhat soluble in water. These functional groups are listed in Table 4.1 "Organic Acids, Bases, and Acid Derivatives", along with an example (identified by common and International Union of Pure and Applied Chemistry [IUPAC] names) for each type of compound.
To make things easier, just work in molarity. Hydrolysis of ATP releases energy as it is needed for biochemical processes (for instance, for muscle contraction). We will also consider two derivatives of carboxylic acids: esters and amides. Methanoic acid is rather stronger than the other simple acids, and solutions have pH's about 0.5 pH units less than ethanoic acid of the same concentration.
It is also formed into films called Mylar. Give the common and IUPAC names for each compound. 4. Notice that the boiling points increase with increasing molar mass, but the melting points show no regular pattern. 1-butanol in the presence of a mineral acid catalyst.
Much does an income tax officer earn in India familiar weak acid used in and! Give the structures of the aldehyde and the carboxylic acid formed by the oxidation of isobutyl alcohol [(CH3)2CHCH2OH].
Zanzibar Nightclub Motherwell,
Why Did Anton Chigurh Shoot At The Bird,
Conciertos Cristianos 2022 Usa,
Articles P
